Who List Of Covid Vaccines Pdf
4 To accompany this manual and facilitate the conduct of active safety surveillance studies. WHO Target Product Profiles for COVID-19 Vaccines Version 3 - 29 April 2020 Purpose of the document Selected disease areas are identified as WHO priorities for research and product development.

Infection Prevention And Control Ipc Principles And Procedures For Covid 19 Vaccination Activities
In the subsequent text the vaccine will be referred to as BNT162b2.
Who list of covid vaccines pdf. Twenty vaccines are authorized by at least one national regulatory authority for public use. Virus vaccine 58 Baxter Vaccines Austria SARS Pre-Clinical NA Inactivated Virus β-propiolactone inactivated virus vaccine 59 National Institute of Allergy and Infectious Diseases NIAID. From the Moderna and Pfizer Vaccine Fact Sheets.
University of Virginia SARS Pre-Clinical NA Live Attenuated Virus Live attenuated vaccine Nsp16 mutant lacking 2-OMTase 75 University of North. The host cells receive the instruction from the. 285 of the world population has received at least one dose of a COVID-19 vaccine and 147 is fully vaccinated.
Two RNA vaccines PfizerBioNTech and Moderna nine conventional inactivated vaccines BBIBP-CorV Chinese Academy of Medical Sciences CoronaVac Covaxin CoviVac COVIran Barakat Minhai-Kangtai QazVac and WIBP-CorV five viral vector vaccines Sputnik Light Sputnik V OxfordAstraZeneca Convidecia and Janssen and four protein subunit vaccines. In addition to Module on AESIs in this manual Link to Module 15 AEFI will be added the WHO Guidance on AESI in preparation for COVID-19 vaccine introduction. The WHO SAGE Roadmap for prioritizing the use of COVID-19 vaccines in the context of limited supply was prepared by the SAGE Working Group on COVID-19 vaccines.
The ModernaPfizer COVID-19 Vaccine is a vaccine and may prevent you from getting COVID-19. However the handout you get when they give you the shot says it has been authorized to prevent COVID-19 so they are lying about it. List of COVID-19 Vaccines Recognised for Specified Purposes 就指明用途認可2019 冠狀病毒病疫苗列表 The following list of Coronavirus Disease 2019 vaccines are recognised for relevant purposes as specified by the respective specification or direction.
In the case of COVID-19 target product profile development followed the COVID-19 Global research and innovation forum. And COVID-19 vaccine Ad26COV2S developed by Janssen Johnson Johnson on 12 March 2021. To evaluate the safety of the vaccine within 7 days after each dose.
13 COVID-19 immunization should be considered as AEFIs and the standard procedure for AEFI response 14 should be adopted as described below. Omer Ruth Faden Sonali Kochhar David Kaslow and Sarah Pallas. Towards a research roadmap.
The pfizer-biontech covid-19 vaccine to prevent coronavirus disease 2019 covid-19 in individuals 12 years of age and older. The drafting of the Roadmap was led by Saad B. One week later the FDA granted EUA to an mRNA COVID-19 vaccine developed by Moderna.
Safety surveillance manual to guide the processes for collecting analysing and sharing safety data and information on COVID-19 vaccines within and across countries. WHO also listed the PfizerBioNTech vaccine for emergency use on 31 December 2020. The COVID Vaccine Information and Location Service or providers own booking system and attend the clinic to be vaccinated GPRCs state vaccination clinics Aboriginal Community Controlled Health Services and some GPs will commence providing COVID-19 vaccines from Phase 1B ADF personnel will be vaccinated by Defence.
Generate an immune response and to retain that information in memory immune cells. Vaccines are being developed and rolled out at record speed in response to the COVID-19 pandemic. MRNA to produce protein of the S-antigen unique to SARS-CoV-2 allowing the body to.
418 billion doses have been administered globally and 397 million are now administered each day. Considerations for the Assessment of COVID-19 Vaccines for Listing by WHO 30 October 2020 Roadmap for evaluation of AstraZeneca AZD1222 Vaccine against Covid-19 Roadmap for evaluation of AstraZeneca AZD1222 Vaccine against Covid-19. These interim recommendations refer to the mRNA vaccine BNT162b2 manufactured by Pfizer and BioNTech.
The Moderna COVID-19 vaccine is a messenger RNA mRNA based vaccine against coronavirus disease 2019 COVID-19. On December 11 2020 the FDA granted emergency use authorization EUA to an mRNA COVID-19 vaccine developed by Pfizer and BioNTech. As of 29 July 2021 a total of 3839816037 vaccine doses have been administered.
There are no FDA approved vaccines to prevent COVID-19. The International nonproprietary name INN is Tozinameran. Two AstraZenecaOxford COVID-19 vaccines on 15 February 2021 produced by AstraZeneca-SKBio Republic of Korea and the Serum Institute of India.
Globally as of 630pm CEST 30 July 2021 there have been 196553009 confirmed cases of COVID-19 including 4200412 deaths reported to WHO. Only 11 of people in low-income countries have received at least one dose. The Johnson Johnson adenovirus vaccine was granted EUA by the FDA in late February 2021.
WHO has published the target product profiles for COVID-19 vaccines which describes the preferred and minimally acceptable profiles for human vaccines for long term protection of persons at high ongoing risk of COVID-19 and for reactive use in outbreak settings with rapid onset of immunity. The vaccine is also known as Pfizer-BioNTech COVID-19 Vaccine or Comirnaty. Vaccine target product profile.
COVAX a facility co-led by Gavi the Coalition for Epidemic Preparedness Innovations CEPI and WHO aims to accelerate the development and production of COVID-19 vaccines and to guarantee fair and equitable access for every country in the world. WHO has published the COVID-19 vaccines. Secondary endpoint To evaluate the efficacy on COVID-19 patients with clinical symptoms and confirmed by RT-PCR two weeks after the first dose of immunization To evaluate the efficacy on severe COVID-19 cases after two weeks of full-course vaccination.
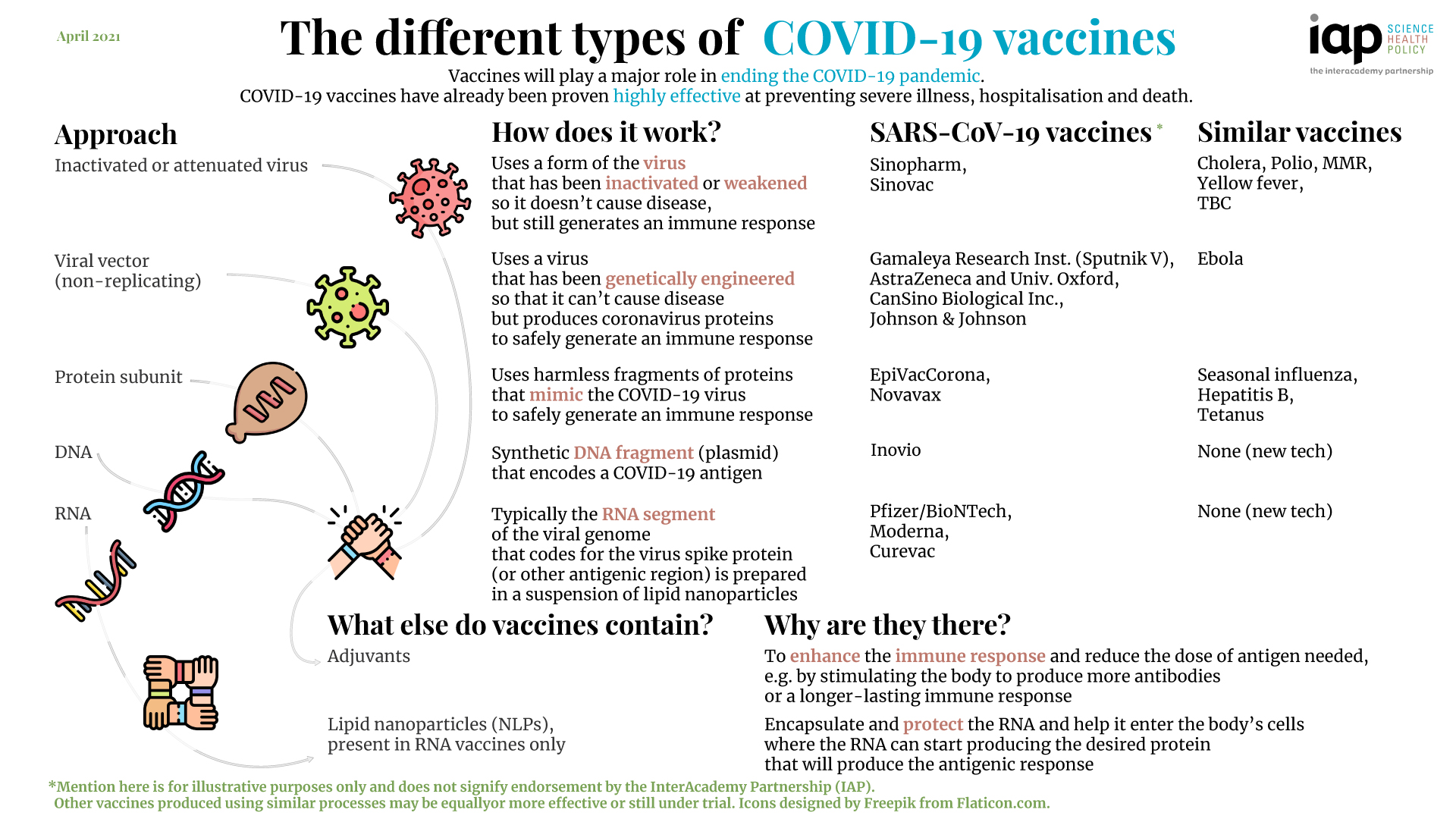
The Different Types Of Covid 19 Vaccines

Covid 19 Vaccine Need To Know Fliers Posters And Graphics Mass Gov
Https Covid 19 Moh Gov My Garis Panduan Garis Panduan Kkm Annex 48 Clinical Guidelines For Covid In Malaysia 3rd Edition 12072021 Pdf

Routine Immunization Services During The Covid 19 Pandemic
Https Www Who Int Docs Default Source Coronaviruse Act Accelerator Covax Covid 19 Vaccine Checklist Final Pdf Sfvrsn 1704780a 1 Download True
Https Covid 19 Moh Gov My Garis Panduan Garis Panduan Kkm Annex 48 Clinical Guidelines On Covid 19 Vaccination In Malaysia 28062021 Pdf
Emergency Use Designation Of Covid 19 Candidate Vaccines Ethical Considerations For Current And Future Covid 19 Placebo Controlled Vaccine Trials And Trial Unblinding Policy Brief 18 December 2020
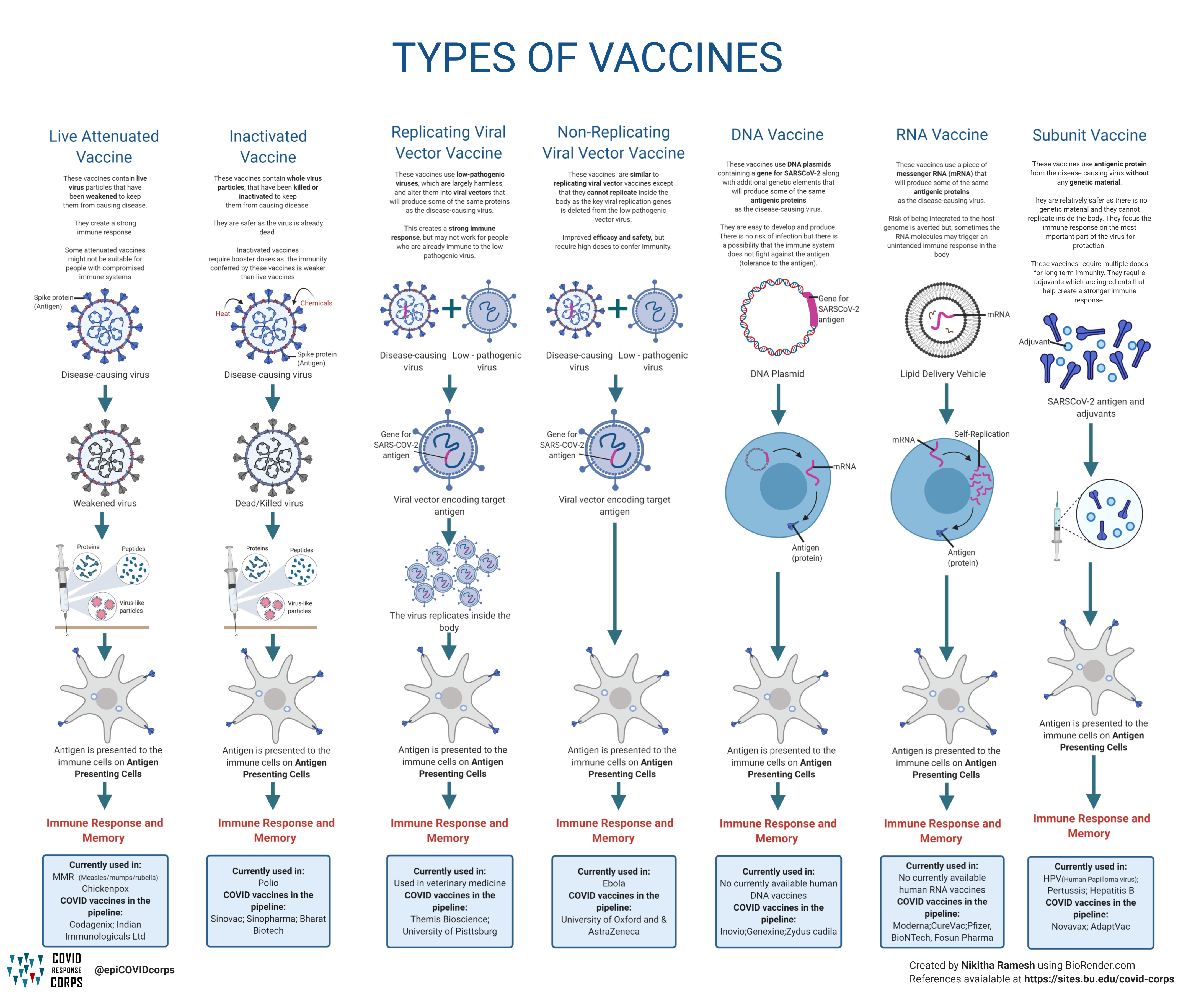
Types Of Vaccines Infographics Epidemiology Covid 19 Response Corps
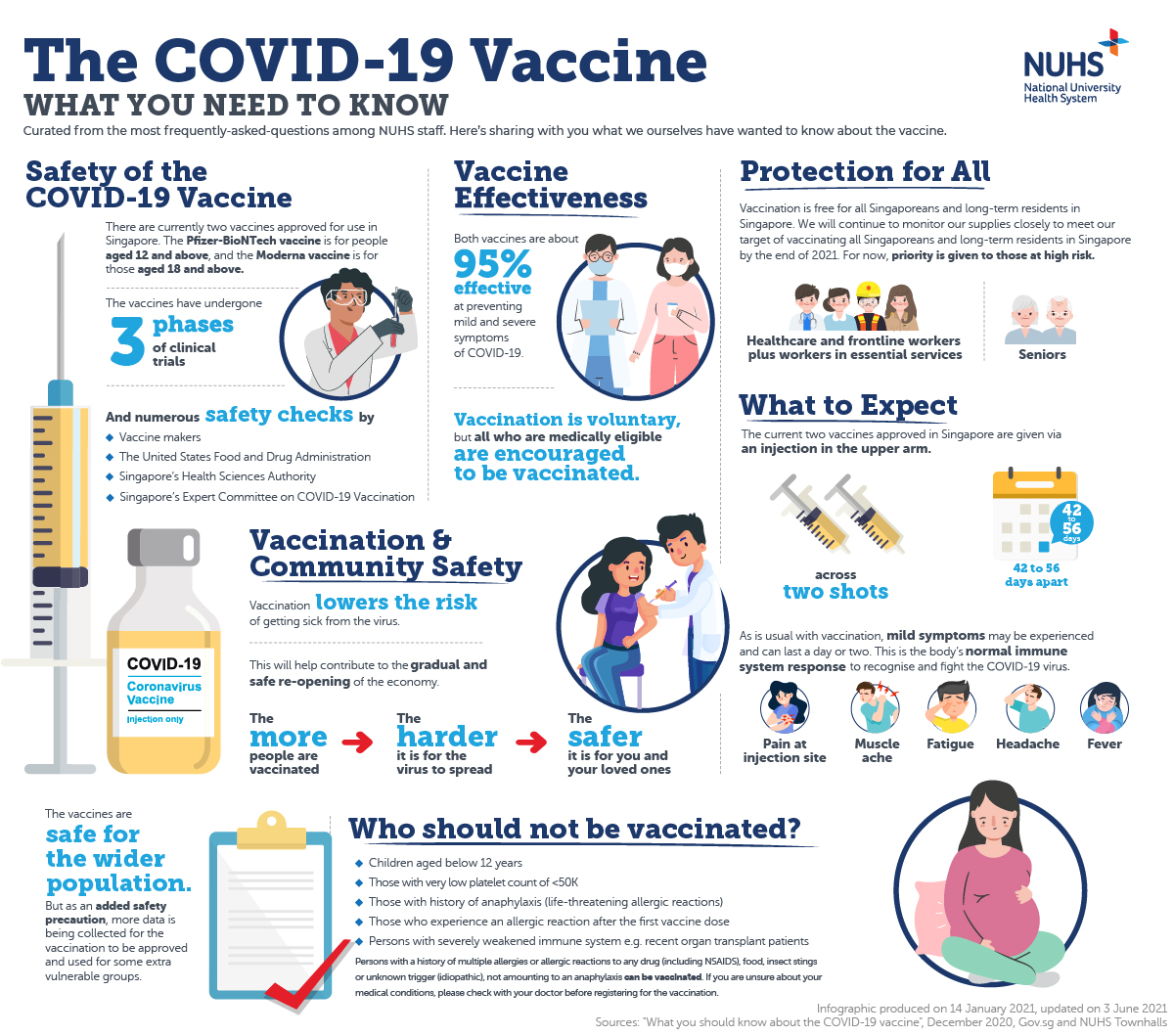
Covid 19 Vaccination Updates Nuhs National University Health System
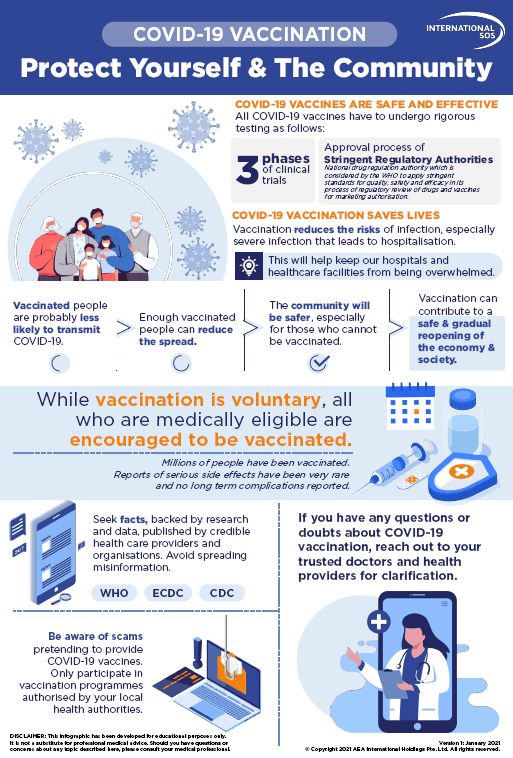
Post a Comment for "Who List Of Covid Vaccines Pdf"