The Covid-19 Vaccine Moderna Requires Dilution
A new study found that the Moderna COVID vaccine is effective for at least six months. Health care providers will need to store it either in dry ice for shorter stints or in specialized freezers.

Britain Gambles On Covid 19 Vaccines Upping The Stakes For The Rest Of Us
Pfizer-BioNTech COVID-19 Vaccine requires mixing ONLY with sterile 09 sodium chloride normal saline preservative-free.

The covid-19 vaccine moderna requires dilution. This page was updated on April 29. More recently reports have described situations in which air has been used accidentally as the diluent for the PfizerBioNTech COVID-19 vaccine. The second dose is necessary and should be given.
COVID-19 Vaccine Pfizer BioNTech Maximum shelf life is 6 months stored in a freezer at -80C to -60C 31 days at 2-8C after thaw assign immediately after removing from freezer In addition once removed from the fridge may be stored between 2 to 25C for 2 hours prior to dilution. It offers 86 protection against the new British strain of COVID-19 which is up to 70 more transmissible. Researchers continue to observe the vaccines efficacy.
CDCs summary table for healthcare professionals on the proper storage preparation and administration of Pfizer-BioNTech Moderna and Janssen Johnson and Johnson COVID-19 vaccines. The new vaccines for coronavirus disease 2019 COVID-19 are highly effective but controversy exists about whether a second dose should be delayed in order to immunize more people. Thaw each vial before use.
Extensive late-stage trials of the Novavax vaccine called NVX-CoV2373 and which also requires two doses suggest it is 89 effective in preventing coronavirus. No other diluent can be used. It may contain white or translucent product-related particulates.
The Pfizer COVID-19 vaccine needs to be stored at minus 70 Celsius. Administer the Pfizer-BioNTech COVID-19 Vaccine intramuscularly. Moderna announced Monday that its vaccine can be stable at refrigerator temperatures for a month and frozen for up to six months.
After dilution vials of Pfizer-BioNTech COVID-19 Vaccine contain six doses of 03 mL of vaccine. This allows the body to generate an antibody response and to retain the information in memory immune cells with the goal of attacking the. The Moderna COVID19 Vaccine uses mRNA to provide a blueprint for your cells to build your bodys defense against the virus.
Once the vial has been defrosted it can be stored at fridge temperature and protected from light for 30 days. Visually inspect the Moderna COVID-19 Vaccine vials for other particulate matter andor discoloration prior to administration. COVID-19 Vaccine plainer Moderna COVID-19 vaccine mRNA-12 7 FEBRUARY 2021 Route and site of administration Intramuscular im administration The preferred site is deltoid muscle.
The Moderna COVID-19 Vaccine is a white to off-white suspension. Pfizer materials have been updated to include the updated Interim Clinical Considerations for the Use of COVID-19 Vaccines Currently Authorized in the United States and the Advisory Committee on Immunization Practices ACIP Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine in Adolescents Aged 1215 Years United States from May 14 2021. Diluent will arrive separately from vaccine in an ancillary supply kit.
Either syringe supplied can be. Do not dilute the vaccine. NEVER use bacteriostatic normal saline or any other diluent.
Dosage 05 mL single dose Diluent None needed Mixing syringe None needed Preparation reconstitution dilution requirement No dilution is required. It will not require dilution at the point of care unlike the. In January 2021 ISMP reported instances in which the PfizerBioNTech COVID-19 vaccine had been improperly diluted with too little diluentor no diluent at all.
The Moderna vaccine does not require dilution. Two doses of the Pfizer and Moderna COVID-19 vaccines are necessary to confer adequate immunity. The contents of the vial must be diluted with 18 mL of sterile 09 Sodium Chloride Injection USP to form the Pfizer-BioNTech COVID-19 Vaccine After dilution the vial contains 5 doses of 03 mL 09 Sodium Chloride Injection USP is not packaged with the vaccine and must be sourced separately.
It can be stored at -25ºC to -15º C for seven months. Moderna Pack volumes may vary check with your supplier he same AZ vaccine is currently being provided in either a green or red capped vial according to production. The Pfizer-BioNTech COVID-19 vaccine multidose vials are intended to yield 6 doses 03 mL of vaccine per vial3 Practice settings have reported that ancillary kits shipped for the purposes of vaccine administration and dilution.

Pfizer Vs Moderna Covid 19 Vaccine What S The Difference Wwlp

Coronavirus Digest Pfizer Moderna Raise Vaccine Prices In Eu News Dw 02 08 2021

Employers Can T Require Covid 19 Vaccination Under An Eua Stat

What To Know About The Pfizer Biontech Covid 19 Vaccine Time

Moderna Says Covid 19 Vaccine Safe And Effective For 12 17 Year Olds Voice Of America English
Japan To Discard Millions Of Pfizer Vaccine Doses Because It Has Wrong Syringes Japan The Guardian
Wrapup 3 Moderna Covid 19 Vaccine Gets The Green Light In Europe Reuters
Covid 19 Vaccination Storage And Supply Chain Strategies February 2021 Pharmacy Purchasing Products Magazine

Your Top Covid 19 Vaccine Questions Answered As Fda Gives The Green Light Shots Health News Npr
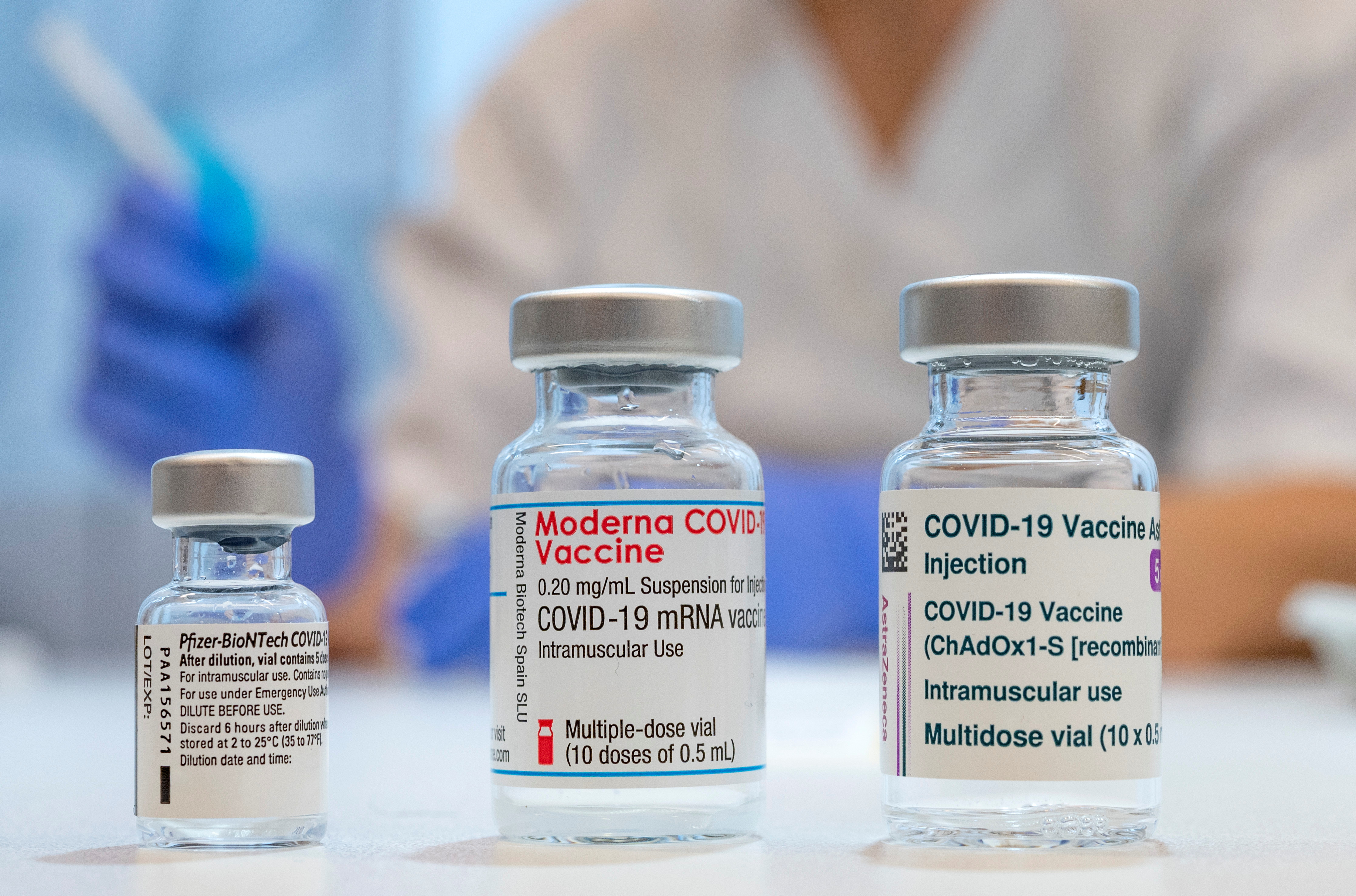
Astrazeneca Sells Stake In Vaccine Maker Moderna For Nearly 1 Billion Reuters
Post a Comment for "The Covid-19 Vaccine Moderna Requires Dilution"