Is Vaccine For Covid 19 Ready In India
The same question is of course valid for being asked of other developing countries in. The US-based company had sought approval in April to conduct a bridging clinical study of its Janssen Covid-19 vaccine candidate in India.
Coronavirus Vaccine Status In India Stages Of Covid 19 Vaccine Development In India
The coronavirus disease 2019 COVID-19 has emerged as a global pandemic with 162 million confirmed cases and 34 million deaths worldwide as on 18 May 2021 As a part of control measures against COVID-19 vaccines have been launched in India from 16 January 2021 which has been subsequently scaled up in response to the surge of cases starting in April 2021.

Is vaccine for covid 19 ready in india. Experts believe that the deadly Covid-19 second wave in India is caused mainly by this variant. Of these 36 are in clinical trials and nine in final states of human trials. Have you come across this claim on the internet yet.
By Gina Krishnan Feb 7 2021 7 min read. Is Indias supply chain ready for the mammoth challenge at hand. The Minister was addressing the FICCI FLO webinar on The Shifting Healthcare Paradigm During and Post.
Reuters Photo Moderna is expecting to launch a single-dose Covid-19 vaccine in India next year and is in talks with Cipla among other Indian firms while another US giant Pfizer is ready. Indias top health agency has reported that the country should have a coronavirus vaccine ready in time for Independence Day on August 15. Nearly nine months into the novel coronavirus pandemic there is a global race to develop a vaccine to control the transmission of the diseaseThere are 182 vaccine candidates in pre-clinical or clinical trials across the world.
For now the deployment has begun with the vaccination of healthcare workers. COVID-19 vaccines to be ready by mid-2021. However according to a new claim circulating on social media India already has secured access to a ready COVID-19 vaccine.
New Delhi May 25. May 25 2021 2114 IST Moderna is expecting to launch a single-dose COVID-19 vaccine in India next year and is in talks with Cipla among other Indian firms while another US giant Pfizer is. Ready set roll-out With two vaccines having received emergency use authorisation India is prepared to meet the logistical challenge.
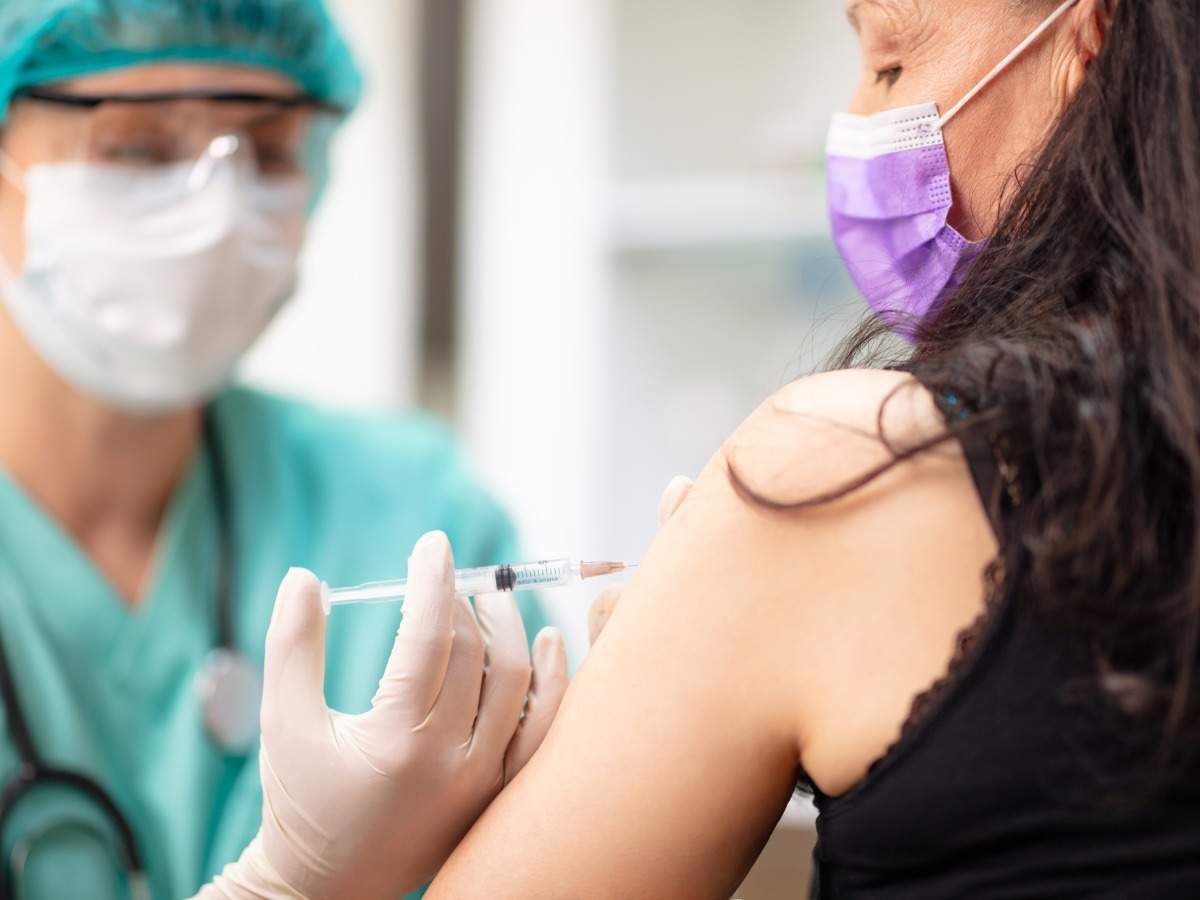
Bharat Biotech had claimed on Monday to have successfully developed Indias first Covid-19 vaccine Covaxin and said it has the permission from the drug controller to start human clinical trials. Now that we are in the second phase of the Covid-19 vaccine deployment in India the big question still remains. COVISHIELD Indias first COVID-19 vaccine is approved safe effective and ready to roll-out in the coming weeks SII CEO Adar Poonawalla said in a.
Moderna is expecting to launch a single-dose COVID-19 vaccine in India next year and is in talks with Cipla among other Indian firms while another US giant Pfizer is ready. It was reported earlier that a few thousand doses of Johnson Johnsons vaccine could arrive in India in July. Amid a raging pandemic and a visible shortage of COVID-19 vaccines who can get vaccinated first needs to be addressed.
With the current shortages of vaccines is India really ready to vaccinate. Union Health and Family Welfare Minister Dr Harsh Vardhan on Thursday said he was confident that the COVID-19 vaccine would be ready in the next three-four months and added that the priority to provide the same to 135 crore Indians would be based on scientific evaluation. Moderna is expecting to launch a single-dose Covid-19 vaccine in India next year and is in talks with Cipla among other Indian firms while another US giant Pfizer is ready to offer 5 crore shots in 2021 itself but it wants significant regulatory relaxations including indemnification sources said on Tuesday.
Biocons Kiran Mazumdar-Shaw As the clamour for a COVID-19 vaccine goes stronger chairperson and managing director of Bengaluru-based Biocon Ltd Kiran. Pfizer has said that it is willing to provide 5crore vaccine doses for those above age 12. Moderna is expecting to launch a single-dose COVID-19 vaccine in India next year and is in talks with Cipla among other Indian firms while another US giant Pfizer is ready to offer 5 crore shots in 2021 itself but it wants significant regulatory relaxations including indemnification sources said.
Moderna is expecting to launch a single-dose COVID-19 vaccine in India next year and is in talks with Cipla among other Indian firms while another US giant Pfizer is ready to offer 5.

India Authorises Moderna S Covid 19 Vaccine For Emergency Use
Brazil Scrambles For India Made Vaccines To Jumpstart Inoculations Reuters

Coronavirus Live India Set For Vaccination Drive Against Covid On Saturday The Economic Times
India Expects Biggest Share Of U S Doses Of Astrazeneca Vaccine Govt Sources Reuters
India S Covaxin To Pfizer Oxford Moderna Latest Updates About Covid Vaccines
100 Million Vaccine Shots Coronavirus Oxford December Adar Poonawalla Serum Institute Of India Astrazeneca S Covid 19 India News India Tv
Coronavirus Vaccine 5 Countries Which Are Leading The Race The Times Of India
Coronavirus Vaccine Update Serum Institute Promises A Vaccine By December And More Updates You Need To Know Today The Times Of India

Coronavirus Vaccines Faqs Cancer Diabetes Hypertension Patients Covid19 Vaccine Approval India News India Tv
Are Asian Countries Choosing Us Or China For The Covid 19 Vaccine South China Morning Post


Post a Comment for "Is Vaccine For Covid 19 Ready In India"