What Is Covid-19 Mrna Vaccine Bnt162b2
Messenger RNA mRNA BNT162b2 Pfizer-BioNTech and mRNA-1273 Moderna COVID-19 vaccines have been shown to be effective in preventing symptomatic COVID-19 in randomized placebo-controlled Phase III trials 1 2. The BNT162b2 vaccine is a lipid nanoparticle-formulated nucleoside-modified messenger ribonucleic acid mRNA vaccine that works to prevent COVID.

Safety And Efficacy Of The Bnt162b2 Mrna Covid 19 Vaccine Ers European Respiratory Society
So you are submitting yourself to a vaccine that has been rushed through putting yourself at risk of side effects and potential disruption to your DNA for something that might not even.

What is covid-19 mrna vaccine bnt162b2. Why am I being given COMIRNATY. COMIRNATY contains the active ingredient BNT162b2 mRNA. The US Food and Drug Administration has authorized two mRNA vaccines to prevent COVID-19.
The SARS-CoV-2 mRNA vaccines either use conventional mRNA or self-amplifying mRNA SAM. BNT162b2 from Pfizer-BioNTech and mRNA-1273 by Moderna are planned for use in mass-immunization programs to curb the pandemic. There are currently three conventional mRNA vaccines in use or in advanced clinical trials that encode the full S protein.
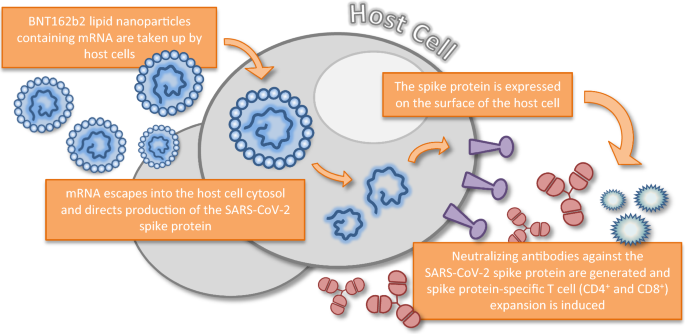
There is a tremendous amount of misinformation about the COVID vaccines and the mRNA vaccines in particularly which then has to be constantly rebutted and debunked. The United States Food and Drug Administration recently issued emergency use authorization for 2 mRNA vaccines for preventing COVID-19 disease caused by SARS-CoV-2 virus infections. Once inside the body the spike protein is produced causing the immune system to recognise it and initiate an immune response.
The PfizerBioNTech COVID-19 vaccine BNT162b2 is an mRNA vaccine encoding a P2 mutant spike protein PS 2 and formulated as an RNAlipid nanoparticle of nucleoside-modified mRNA modRNA. For more information see Section 1. Added revised protocol.
This protocol is for the administration of coronavirus COVID-19 mRNA Vaccine BNT162b2 by appropriately trained persons. The BNT162b2 mRNA COVID-19 vaccine Pfizer-BioNTech on 11 December 2020 and the mRNA-1273 COVID-19 vaccine Moderna on 18 December 2020. BNT162b2 is a lipid nanoparticle-formulated nucleoside-modified RNA vaccine that encodes a prefusion stabilized membrane-anchored SARS-CoV-2 full-length spike protein.
It is required to have two injections given 21 days apart BUT they admit that it may not fully protect all those who receive it. It contains the genetic code mRNA of the spike protein which is found on the surface of the SARS-CoV-2 virus. The primary end points were efficacy of the vaccine against laboratory-confirmed Covid-19 and safety.
Updated 25 June 2021 pursuant to updated interim recommendations WHOs Strategic Advisory Group of Experts on Immunization SAGE has issued its policy recommendations for the rollout of the first COVID-19 vaccine approved for emergency use the Pfizer-BioNTech COVID-19 vaccineAccording to SAGE the Pfizer-BioNTech COVID-19 mRNA vaccine is safe and effective. The COVID-19 mRNA Vaccine BNT162b2 is given as an injection of 03 mL in the upper arm. Added version 3 details of changes on page 3.
The BNT162b2 vaccine is a COVID-19 mRNA vaccine developed by Pfizer and BioNTech. Unfortunately the complexity of COVID mRNA immunity and vaccines is such that those who wish to raise fears about the vaccine can exploit partial information. However the benefits of these vaccines for preventing asymptomatic and symptomatic SARS-CoV-2 the virus that causes COVID-19 infection particularly.
This study in a nationwide mass vaccination setting suggests that the BNT162b2 mRNA vaccine is effective for a wide range of Covid-19related outcomes a finding consistent with that of the. The mRNA vaccinesModernaCOVID-19 Vaccine mRNA-1273 and Pfizer-BioNTechCOVID-19 BNT-162b2are administered as intramuscular injections Both of the mRNA vaccines require 2 doses ModernaCOVID-19 Vaccine-Give 2 doses each 05 mL-Give 1 month 28 days apart-Each dose contains 100 µg mRNA. COMIRNATY is a vaccine given to prevent coronavirus disease 2019 COVID-19 caused by severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 in adults and adolescents from 16 years of age and older.
These are the mRNA-1273 vaccine by Moderna BNT162b2Comirnaty by BioNTechPfizer and CVnCoV by CureVac Table 1. BNT162b2 elicits a blunted innate immune sensor activating capacity and thus augments antigen expression. In the full CMI.
Covid 19 Pfizer Vaccine Archives Rebel Em Emergency Medicine Blog
Pfizer Biontech Sars Cov 2 Vaccine Effective For Wide Range Of Covid 19 Related Outcomes
Bnt162b2 Covid 19 Vaccine Is 95 Percent Effective Finds Final Analysis
Bnt162b2 Mrna Covid 19 Vaccine First Approval Springerlink

Inside The Hunt For A Covid 19 Vaccine How Biontech Made The Breakthrough Financial Times

Safety And Efficacy Of The Bnt162b2 Mrna Covid 19 Vaccine Nejm
Biontech Vaccine Mass Production In China Close To Ready May Get Go Ahead Before July Global Times

Side Effects Of Bnt162b2 Mrna Covid 19 Vaccine A Randomized Cross Sectional Study With Detailed Self Reported Symptoms From Healthcare Workers International Journal Of Infectious Diseases
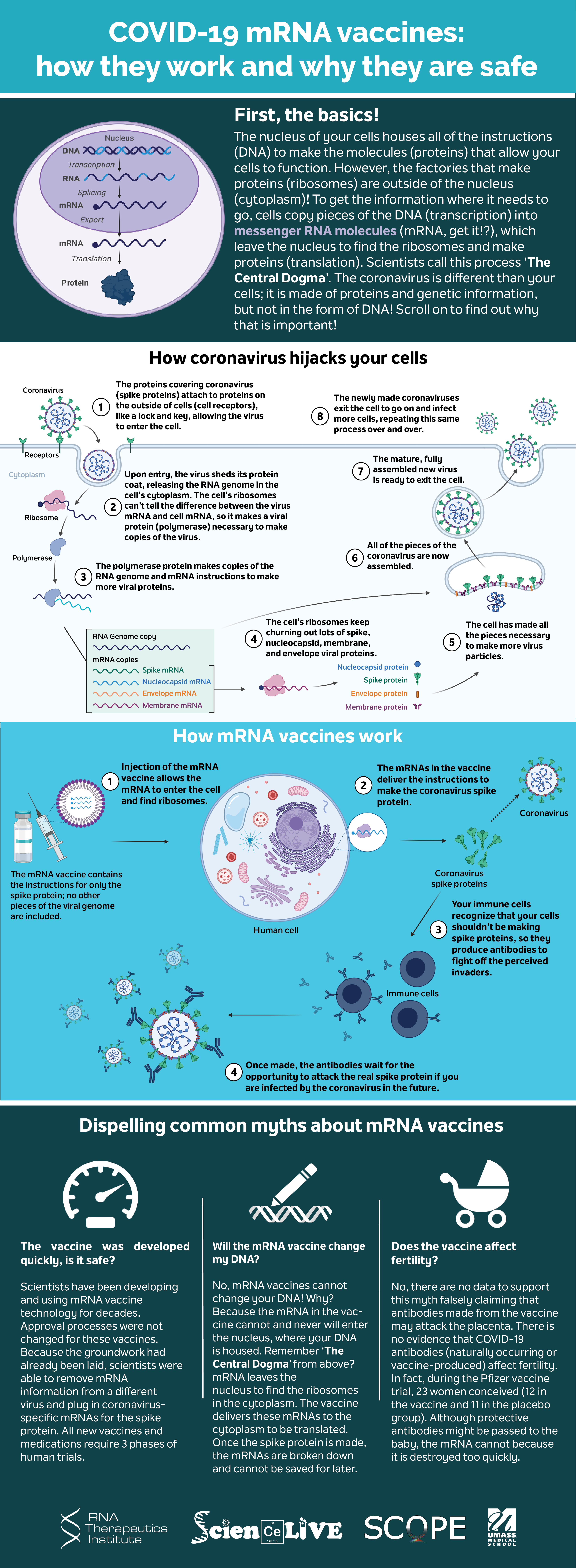
Covid 19 Mrna Vaccines Explained
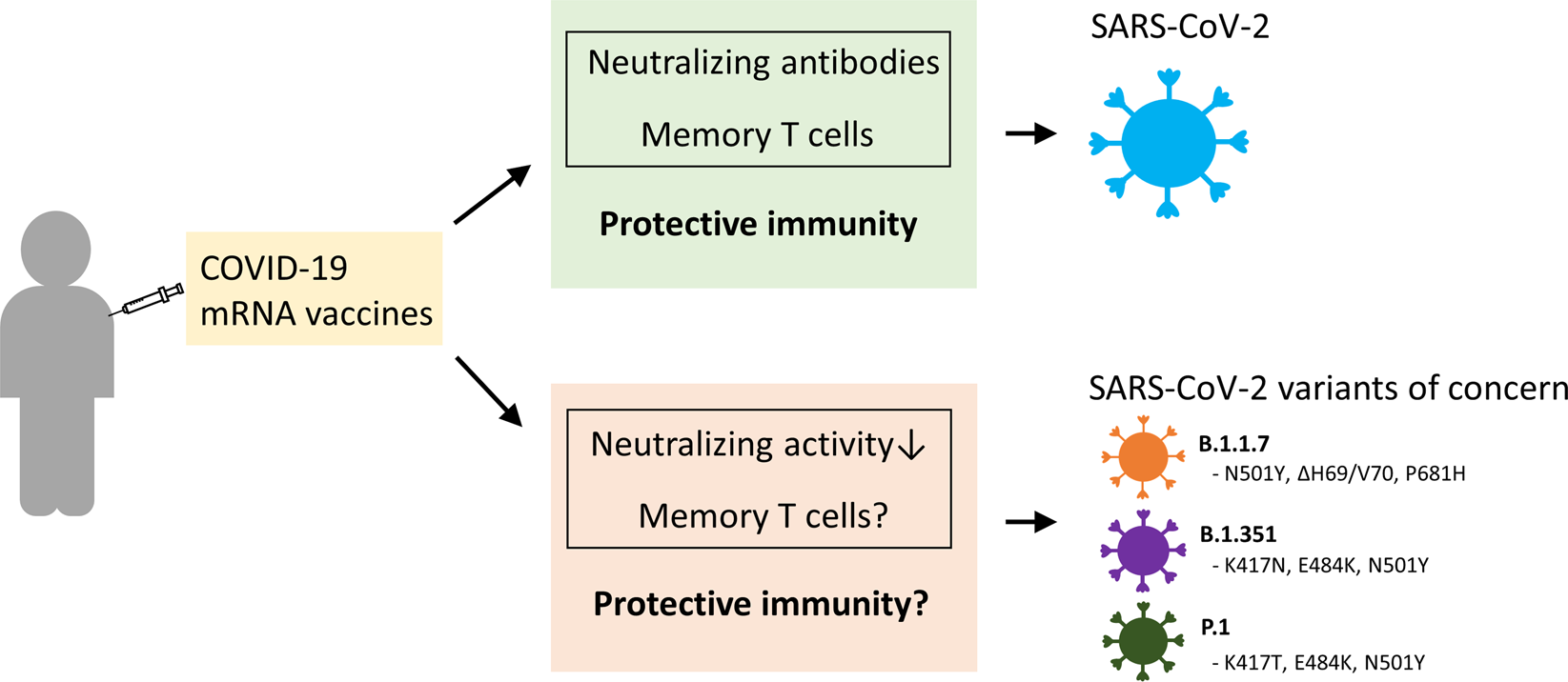
Sars Cov 2 Mutations Vaccines And Immunity Implication Of Variants Of Concern Signal Transduction And Targeted Therapy
Post a Comment for "What Is Covid-19 Mrna Vaccine Bnt162b2"