Moderna Covid Vaccine Study Results
M oderna said Monday that its Covid-19 vaccine continued to deliver strong efficacy results. Pfizer Vaccine BNT162b2 Results of Phase 3 study of mRNA-based COVID-19 vaccine candidate BNT162b2 met all of the studys primary efficacy endpoints.
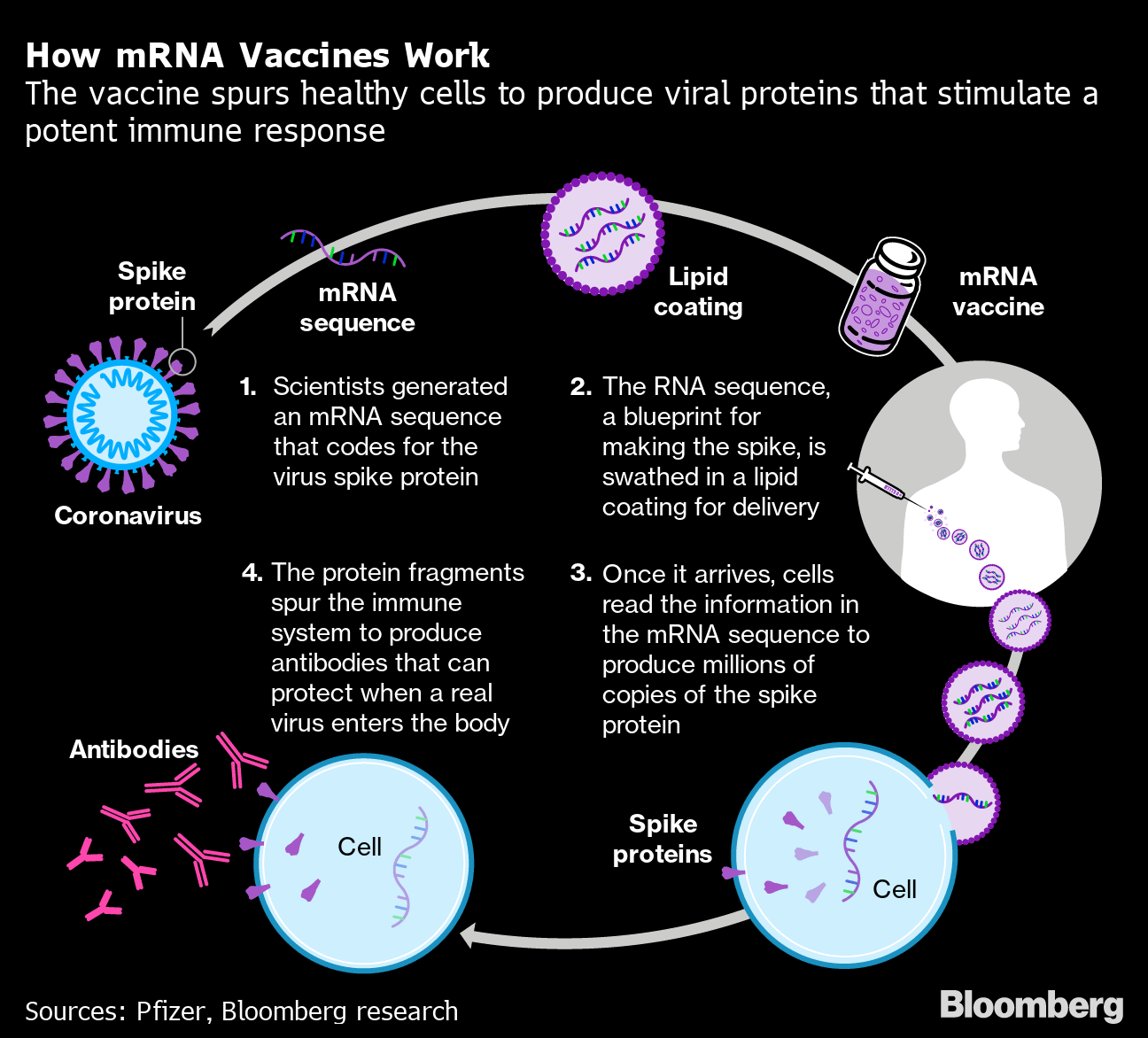
Moderna Mrna Vaccine Is Found Highly Effective At Preventing Covid 19 Bloomberg
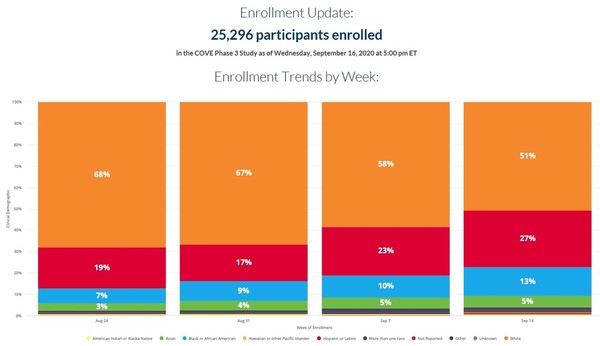
The trial enrolled 30420 volunteers who were randomly assigned in a 11 ratio to receive either vaccine or placebo 15210 participants in each group.
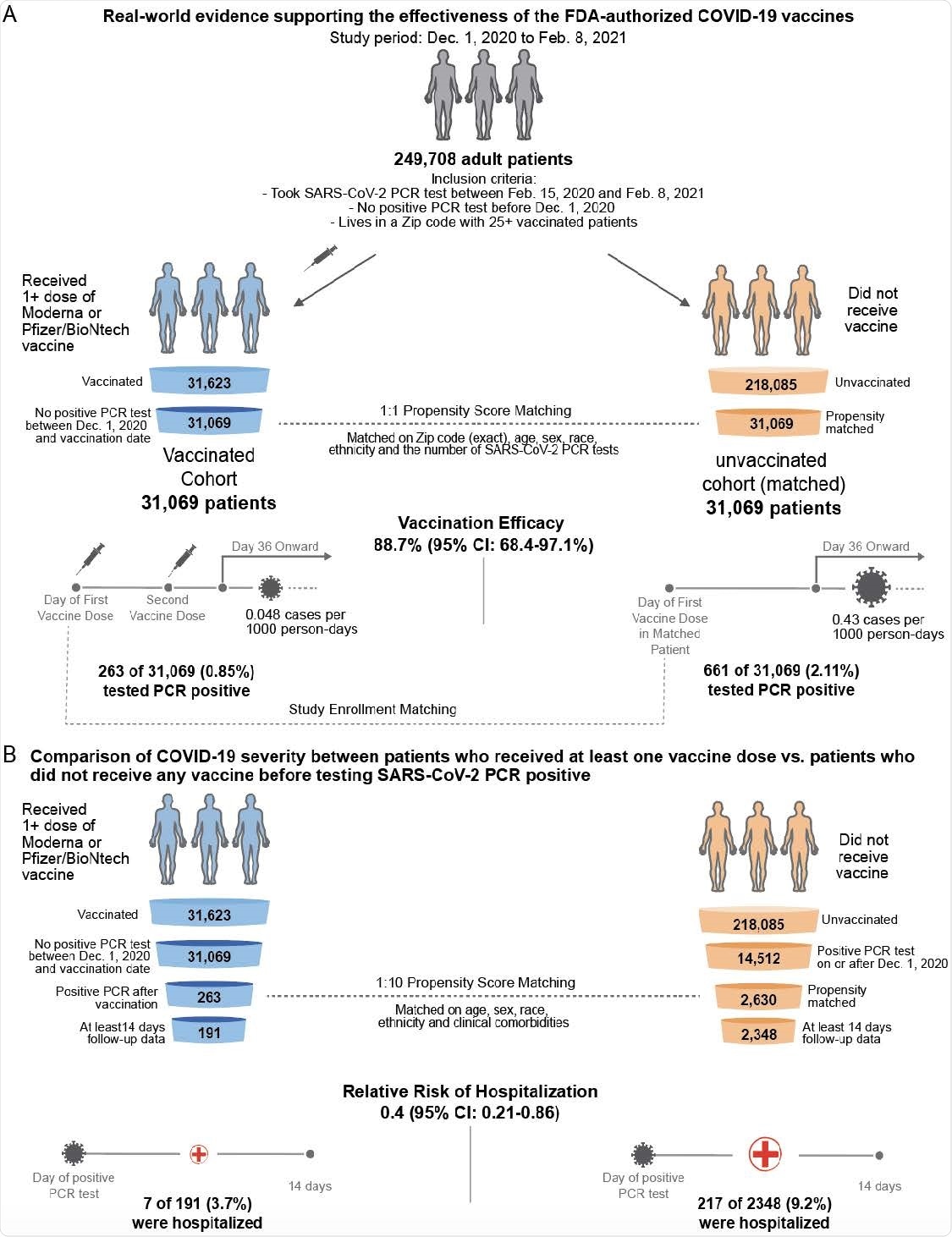
Moderna covid vaccine study results. The Janssen COVID-19 vaccine also referred to as the Johnson Johnson vaccine or Ad26COV2-S the Moderna COVID-19 vaccine also known as mRNA-1273 and the Pfizer-BioNTech COVID-19 vaccine. Analysis of the data indicates a vaccine efficacy rate of 95 p. In a study published in the journal Nature researchers discovered that the vaccines made by Pfizer-BioNTech and Moderna set off a persistent immune reaction in.
It is authorized for use in people aged 12 years and older in some. 28 2021 Modernas vaccine appears to have a higher rate of side effects with about 74 percent of V-safe respondents reported. Recognizing the disproportionate impact of the epidemic on underrepresented minority populations investigators worked with.
Repeated antibody measurements from before the patients vaccination to 60 days after the second dose also shows significantly delayed or suboptimal responses particularly in patients who have not contracted COVID-19. Said its Covid-19 vaccine was effective in children aged 12 to 17 in a new study a finding that could clear the way for a second shot for use in adolescents. More than 30000 participants at 100 clinical research sites in the United States are participating in the study which launched on July 27 2020 after results from earlier stage clinical testing indicated that the vaccine candidate is well-tolerated and immunogenic.
Elasomeran codenamed mRNA-1273 and sold under the brand name Spikevax is a COVID-19 vaccine developed by Moderna the United States National Institute of Allergy and Infectious Diseases NIAID and the Biomedical Advanced Research and Development Authority BARDA. In a study published online in MMWR the CDC reported that people who had received both doses of either mRNA vaccine PfizerBioNTech or Moderna were 90 less likely to get infected with COVID-19 than people who were not vaccinated. In clinical trials the vaccines were 95 PfizerBioNTech and 941 Moderna effective.
At the FDAs urging Pfizer-BioNTech and Moderna are expanding their trials for children 5 to 11. This study underscores the need for routine blood tests on multiple myeloma patients after vaccination to understand their risk and the potential need to continue. Marisol Gerardo 9 received a second dose of the Pfizer-BioNTech Covid-19 vaccine during a.
The lowest responses were in the 25-μg dose group with a geometric mean ID 50 of 1123 95 CI 712 to 1771 at day 43. In a release on Tuesday June 29 Moderna announced that the new results from its in vitro neutralization studies of sera from individuals vaccinated with the Moderna COVID-19 vaccine has produced neutralizing titers against all variants tested including the Beta variant B1351 first identified in South Africa three lineage variants of B1617 first identified in India that includes the Delta variant. The company reported results from its combined Phase 2 and 3 study.
Looking at this data gathered from Dec. Based on evidence from clinical trials in people aged 18 years and older the Moderna vaccine was 941 effective at preventing laboratory-confirmed COVID-19 infection in people who received two doses and had no evidence of being previously infected. I n a release on May 25 Moderna says its vaccine is safe and efficacious among 12- to 18-year olds.
14 2020 to Feb. The higher responses in the 100-μg and 250-μg groups were similar in. Moderna to submit Covid-19 vaccine to FDA as full results show 94 efficacy.
Final results from the trials of Modernas vaccine against Covid-19 confirm it has 94 efficacy and nobody who was vaccinated with it developed severe disease said the company kickstarting the. The Moderna COVID19 vaccine pINN. The Cambridge Mass.
It will include approximately 150 individuals who already have received one of the three COVID-19 vaccine regimens currently available under FDA Emergency Use Authorization in the United States.

First Data For Moderna Covid 19 Vaccine Show An Immune Response Stat
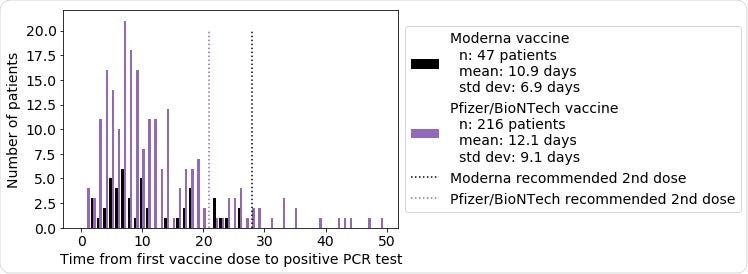
Study Shows Real World Effectiveness Of Moderna And Pfizer Biontech Vaccines
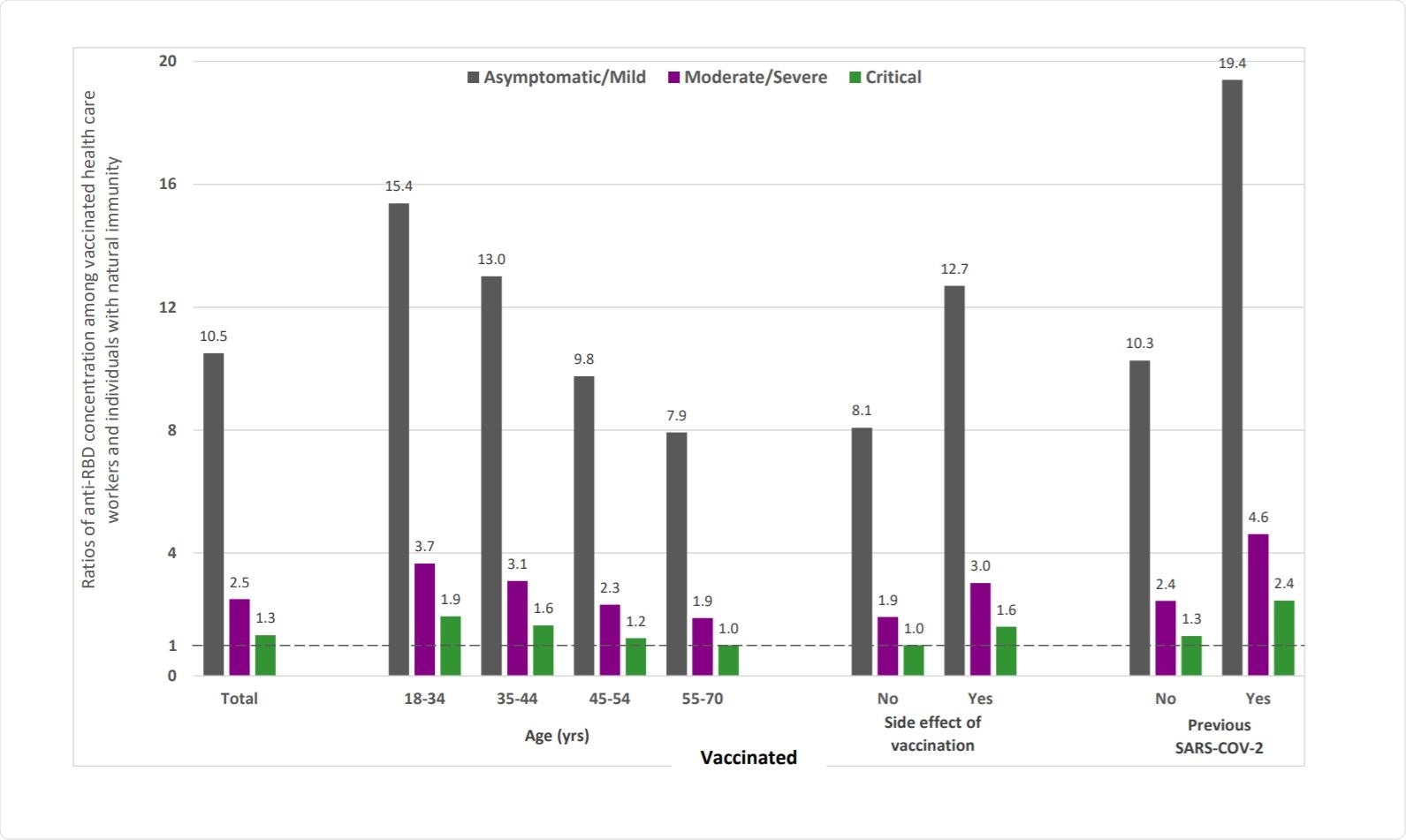
Higher Levels Of Antibodies From Covid 19 Mrna Vaccine Compared To Natural Sars Cov 2 Infection

Covid 19 Safety And Efficacy Of The Mrna 1273 Sarscov 2 Vaccine Moderna Against Sars Cov 2
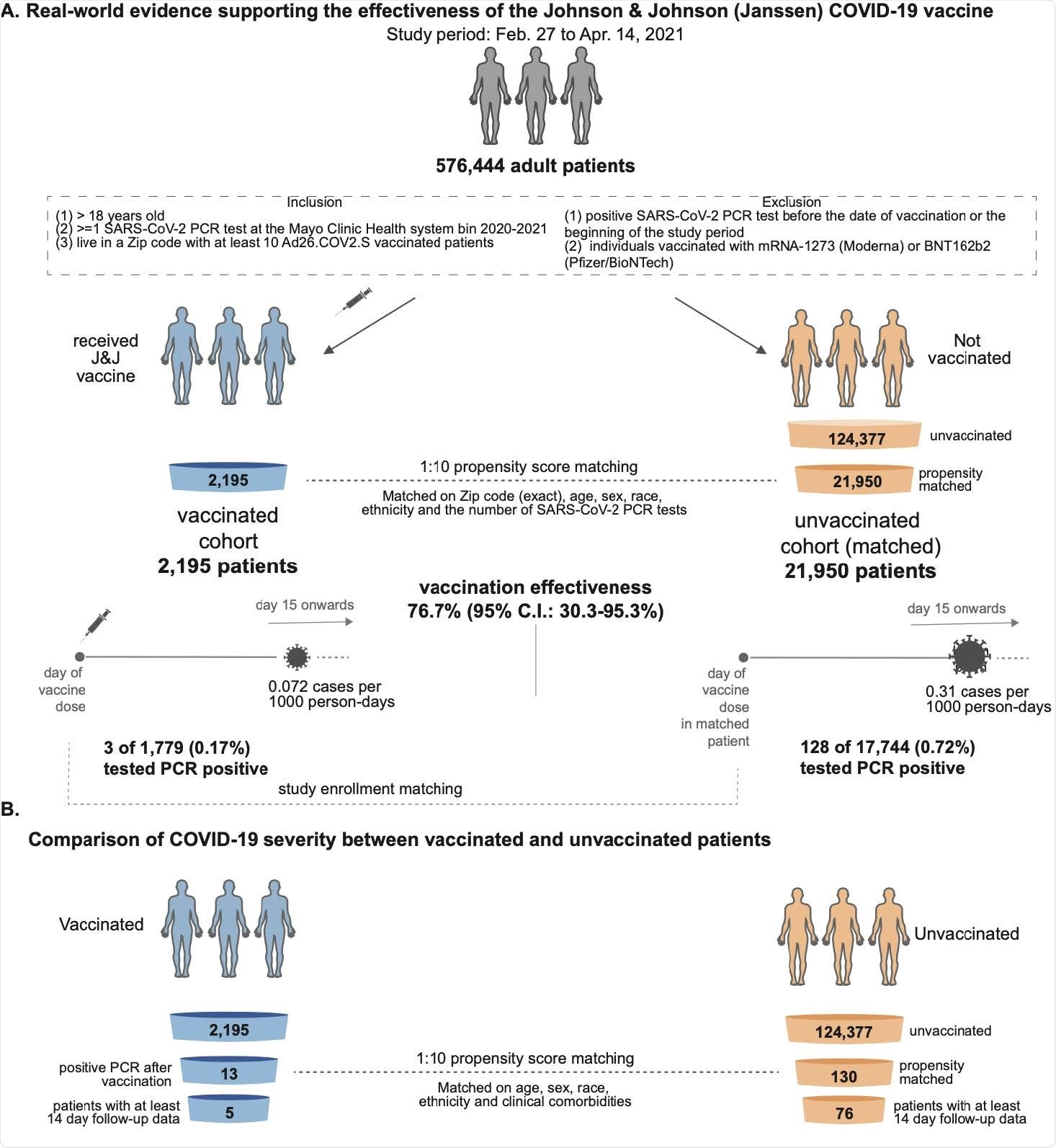
Researchers Demonstrate Real World Effectiveness Of Johnson Johnson Covid 19 Vaccine
Moderna Says Its Coronavirus Vaccine Is More Than 94 Effective
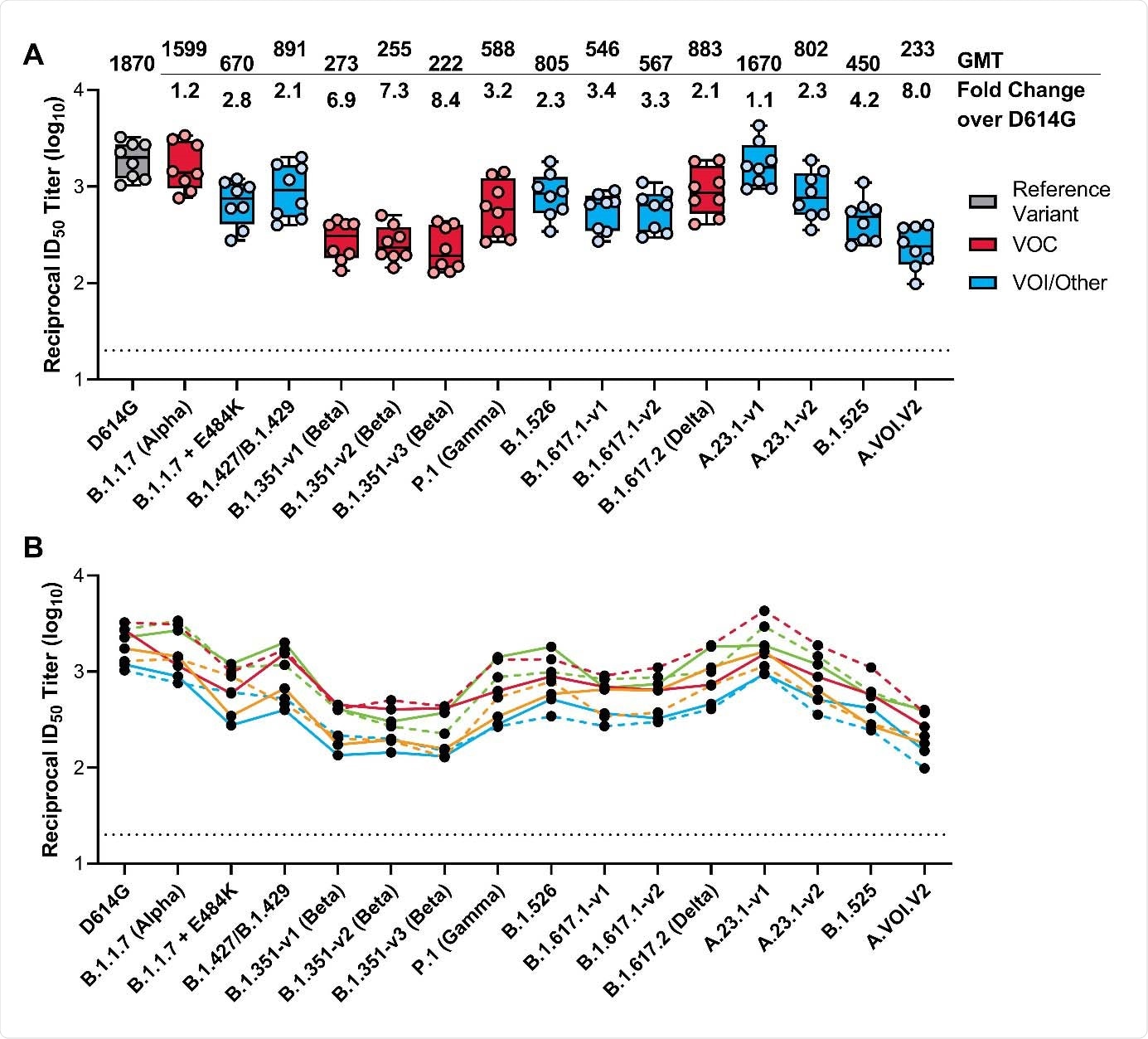
Moderna S Covid Vaccine Effective Against Emerging Variants Including Delta
The Economist Phase Three Trials Of China S Coronavac Vaccine Which Were Conducted On Health Care Workers In Brazil Yielded An Efficacy Rate Of Just 50 7 This Is Barely Above The 50 Threshold Set

Study Shows Real World Effectiveness Of Moderna And Pfizer Biontech Vaccines

Post a Comment for "Moderna Covid Vaccine Study Results"