Covid-19 Vaccine Mrna-1273
Moderna COVID-19 mRNA-1273 vaccine July 2021. MRNA-1273 has not yet been established.

Covid 19 Safety And Efficacy Of The Mrna 1273 Sarscov 2 Vaccine Moderna Against Sars Cov 2
Here is what you need to know.
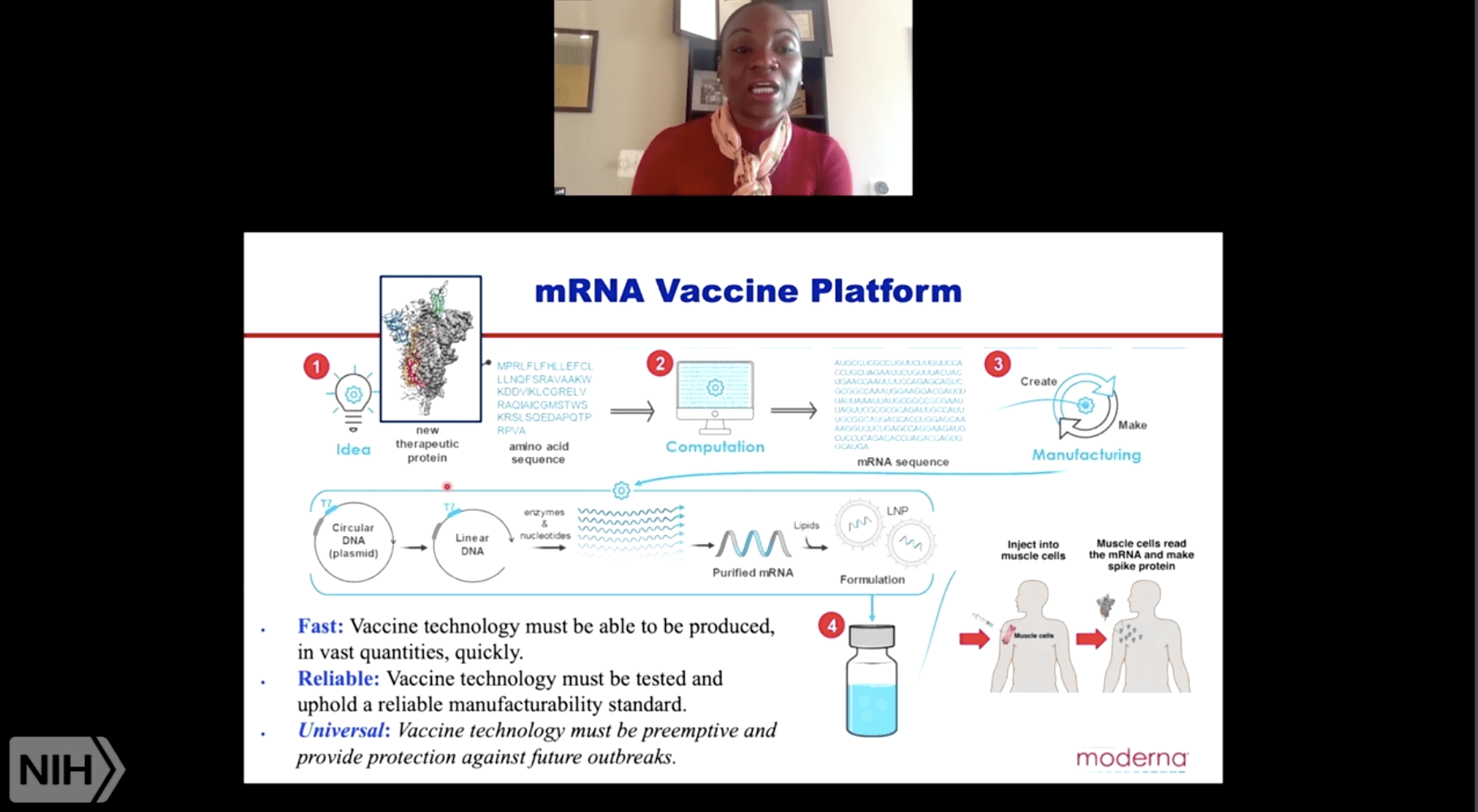
Covid-19 vaccine mrna-1273. Modernas mRNA-1273 COVID-19 vaccine is a LNP-encapsulated mRNA vaccine expressing the prefusion-stabilized spike glycoprotein and was developed by Moderna and the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases NIAID. The United States Food and Drug Administration recently issued emergency use authorization for 2 mRNA vaccines for preventing COVID-19 disease caused by SARS-CoV-2 virus infections. Listing a study does not mean it has been evaluated by the US.
Food and Drug Administration FDA authorized the emergency use of mRNA-1273 to prevent COVID-19 in individuals 18 years of age and older on December 18 2020. MRNA-1273 Vaccine to Prevent Covid-19 Two injections of mRNA-1273 a lipid nanoparticleencapsulated mRNA-based vaccine produced in collaboration with the NIAID that encodes the SARS-CoV-2 spike. The mRNA-1273 vaccine popularly called the Moderna vaccine is being widely administered in the United States for the prevention of COVID-19 infection since December 2020.
MRNA to produce protein of the S-antigen unique to SARS-CoV-2 allowing the body to. Fact sheet for health workers. COVID-19 mRNA vaccines give instructions for our cells to make a harmless piece of what is called the spike protein The spike protein is found on the surface of the virus that causes COVID-19.
MRNA-1273 is an investigational mRNA vaccine against SARS-CoV-2 in development for the prevention of COVID-19. Potential adverse impacts due to the global COVID-19 pandemic such as delays in clinical trials preclinical work overall operations regulatory review manufacturing and supply chain interruptions adverse effects on healthcare systems and disruption of the global economy. First COVID-19 mRNA vaccines are given in the upper arm muscle.
Updated 25 June 2021 pursuant to updated interim recommendations The WHO Strategic Advisory Group of Experts SAGE on Immunization has issued Interim recommendations for use of the Moderna mRNA-1273 vaccine against COVID-19 in people aged 18 years and olderHere is what you need to knowWho should be vaccinated firstAs with all COVID-19 vaccines health workers at high risk of exposure and older people should be prioritized for vaccinationAs more vaccine. Safety and Immunogenicity Study of 2019-nCoV Vaccine mRNA-1273 for Prophylaxis of SARS-CoV-2 Infection COVID-19 The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. What you need to know.
The doses are administered 28 days apart and the vaccine trains the. Standard curves for mRNA-1273 Moderna and BNT162b2 Pfizer vaccines were generated to enable calculation of COVID-19 vaccine mRNA concentration in postvaccination human milk samples eMethods in the Supplement. The host cells receive the instruction from the.
Information is limited regarding the effectiveness of the two-dose messenger RNA mRNA vaccines BNT162b2 Pfizer-BioNTech and mRNA-1273 Moderna in preventing infection with severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 and in attenuating coronavirus disease 2019 Covid-19 when administered in real-world conditions. Moderna COVID-19 mRNA-1273 vaccine July 2021. Regional Office for Europe.
Generate an immune response and to retain that information in memory immune cells. Findings In this retrospective cohort study of US veterans with cirrhosis that compared 20 037 patients who received either a Pfizer BNT162b2 mRNA or a Moderna mRNA-1273 COVID-19 vaccine with 20 037 propensity score matched controls receipt of 1 dose of either vaccine was associated with a 648 reduction in COVID-19 infections and 100 reduction in hospitalization or death due to COVID. The Moderna COVID-19 vaccine is a messenger RNA mRNA based vaccine against coronavirus disease 2019 COVID-19.
BNT162b2 from Pfizer-BioNTech and mRNA-1273 by Moderna are planned for use in mass-immunization programs to curb the pandemic. Mild to moderate intensity side effects like low-grade fever myalgia chills and malaise were reported in the trials related to the vaccine. Modernas COVID-19 vaccine also known as mRNA-1273 is a two-dose vaccine.
Factsheet for health workers. The Moderna COVID-19 mRNA-1273 vaccine. The WHO Strategic Advisory Group of Experts SAGE on Immunization has issued Interim recommendations for use of the Moderna mRNA-1273 vaccine against COVID-19 in people aged 18 years and older.
Based on 95 cases of which 90 cases of COVID-19 were observed in the placebo group versus 5 cases observed in the mRNA-1273 group resulting in a point estimate of vaccine efficacy of 945 p. B mRNA-1273 or BNT162b2 vaccines were inoculated into prevaccine milk samples to assess the effect of a single freezethaw cycle on the vaccine mRNA. MRNA-1273 is an mRNA vaccine that works by using snippets of a.

Moderna Mrna 1273 Covid 19 Vaccine
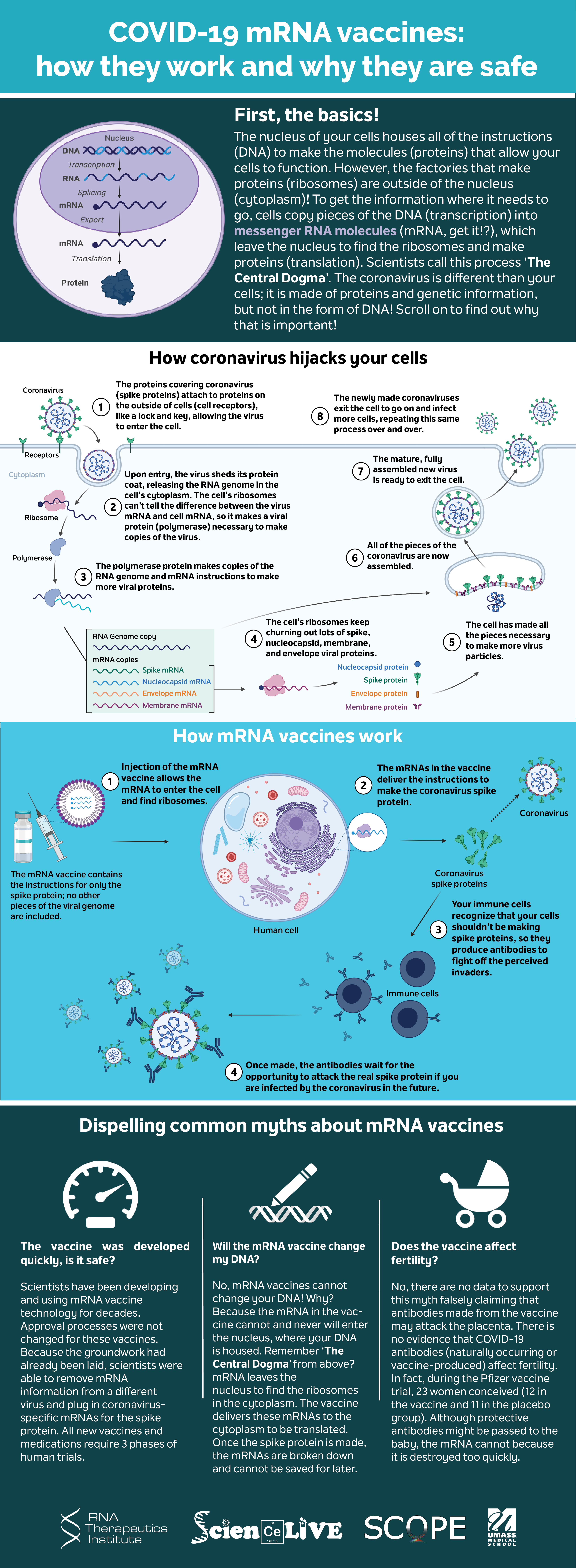
Covid 19 Mrna Vaccines Explained

Moderna Says Covid 19 Boosters Effective Against Variants
Moderna Vaccine Moderna Covid 19 Vaccine Side Effects Doses Price Efficacy Times Of India
Switzerland Starts Accelerated Review Of Moderna Covid 19 Vaccine
Cipla Seeks Dcgi Nod To Import Moderna S Covid 19 Vaccine Decision Likely Today Latest News India Hindustan Times

Covid 19 Safety And Efficacy Of The Mrna 1273 Sarscov 2 Vaccine Moderna Against Sars Cov 2

Nih Covid 19 Lecture On Sars Cov 2 Mrna Vaccine Immunopaedia
The New England Journal Of Medicine Quick Take A Covid 19 Mrna Vaccine In Older Adults Facebook

Covid 19 Vaccine Mrna 1273 Elicits A Protective Immune Profile In Mice That Is Not Associated With Vaccine Enhanced Disease Upon Sars Cov 2 Challenge Sciencedirect



Post a Comment for "Covid-19 Vaccine Mrna-1273"